West Coast States Break with CDC, Issue Independent Vaccine Guidelines
California, Oregon, Washington, and Hawaii form health alliance to recommend broader flu, COVID-19, and RSV vaccinations amid federal uncertainty.
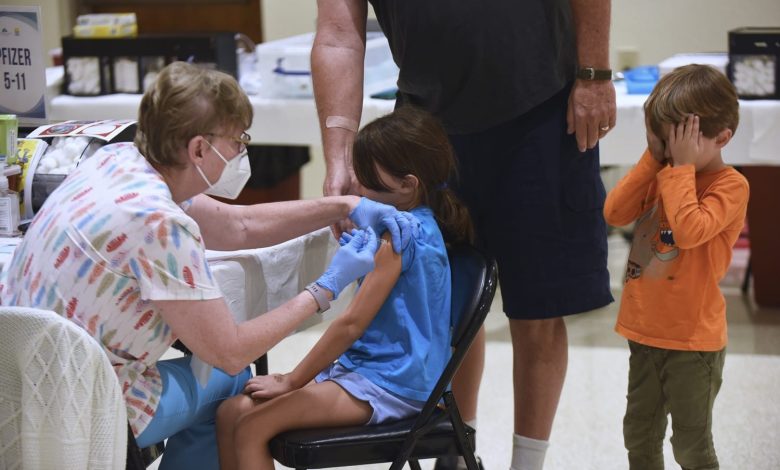
In an unprecedented step that reflects open disagreement with federal health policy, four Democrat-led states — California, Oregon, Washington, and Hawaii — announced their own seasonal vaccine recommendations under what they call the West Coast Health Alliance.
The new guidelines call for flu vaccines for everyone starting at six months of age, broad use of COVID-19 vaccines, and targeted RSV (respiratory syncytial virus) vaccines for the most vulnerable groups, including infants, the elderly, and people with chronic health conditions.
These recommendations closely mirror guidance from leading medical bodies such as the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians. However, they diverge from the current federal line: the Centers for Disease Control and Prevention (CDC), led by Health Secretary Robert F. Kennedy Jr., recently scaled back COVID-19 vaccine guidance, particularly for pregnant women and young children.
West Coast Alliance Issues Expanded Vaccine Guidance
The alliance explained that the move aims to protect populations and reduce pressure on hospitals during the winter season, at a time when the CDC faces uncertainty following the dismissal of all members of the Advisory Committee on Immunization Practices (ACIP) and their replacement with new appointees, some known for questioning vaccine safety.
Details of the recommendations include COVID-19 vaccines for all children aged 6–23 months, and for children 2–18 years with risk factors or who live with vulnerable individuals. The vaccine is also advised for pregnant or breastfeeding women, adults over 65, and younger adults with chronic conditions.
RSV vaccines were specifically recommended for high-risk groups: infants under 8 months, pregnant women between 32–36 weeks, seniors over 75, and adults aged 50–74 with serious health conditions.
The move has created visible tension with CDC guidance, leading to confusion on the ground. In Oregon, some pharmacies required prescriptions for COVID-19 booster doses, prompting an emergency meeting of the state Board of Pharmacy to consider allowing pharmacists to administer vaccines directly without prescriptions.
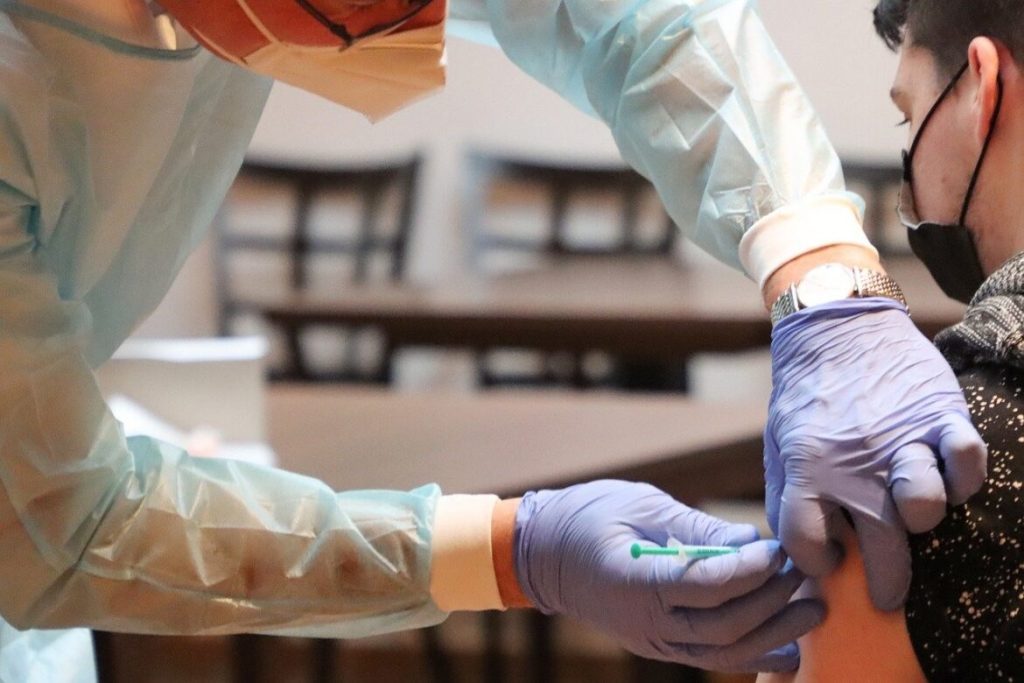
Federal and State Health Leaders Clash Over Vaccine Guidance
Dr. Erica Pan, California’s Public Health Director, stressed that the goal is to provide clear, science-based information to the community, away from federal ambiguity. She said: “There is a health and science community that will always stand together for the public good.”
Meanwhile, the Senate Health Committee heard testimony from former CDC director Susan Munariz, who declared: “I would never support guidelines that lack scientific evidence.”
For its part, the Department of Health and Human Services emphasized through spokesperson Andrew Nixon that ACIP remains the nationally authorized scientific body for immunization guidance, adding: “We will ensure that health policies are based on solid science, not on the failed politics of the pandemic era.”
The newly restructured CDC advisory committee is expected to meet Thursday and Friday to review recommendations on COVID-19, hepatitis B, and measles vaccines — a move that could reshape national immunization policy in the United States.



